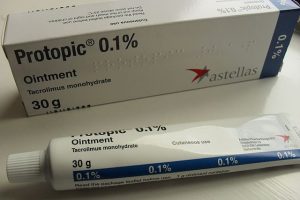
Dermatologists often prescribe two creams containing tacrolimus (Protopic®) or pimecrolimus (Elidel®) for children or adults with atopic dermatitis. These drugs belong to the group of calcineurin inhibitors, which are considered as a second option for those who have been using corticosteroid creams for a long time or these have not worked adequately.
Although we doctors prescribe them with peace of mind (because they are accepted in all atopic dermatitis treatment guidelines), the reality is that the package insert of these two creams contains “controversial” parts that can undoubtedly frighten those who use them for the first time:
“Cases of malignant disease, including cutaneous malignant disease (e.g. cutaneous T-cell lymphomas) and other types of lymphoma, and skin cancers have been described in patients using tacrolimus ointment.”
But should we be so afraid of them? I anticipate that we should not be, but I will now explain one by one the different aspects of this treatment for atopic dermatitis…
How do tacrolimus and pimecrolimus work?
Both tacrolimus and pimecrolimus slow down the action of a molecule called calcineurin, which is found inside skin cells. When this molecule does not perform its function, other cells, the T lymphocytes involved in dermatitis, are prevented from being activated, thus preventing or reducing the occurrence of flare-ups.
When should they be used?
Pimecrolimus and Tacrolimus are excellent creams for mild to moderate atopic dermatitis. We usually use them specifically for the face, neck, creases (armpits, groin) or genital area. Corticosteroid creams and calcineurin inhibitors have very similar effects. Therefore, we should not forget that corticosteroid creams are still the mainstay of treatment, as discussed in this recent article in the JAAD (Journal of the American Academy of Dermatology).
Calcineurin inhibitors “save corticosteroids”.
The most common side effects of pimecrolimus and tacrolimus are redness and burning after application. These effects do not occur with corticosteroids, which on the other hand are much cheaper. Thus, calcineurin inhibitors are now considered second-line agents, but they are very useful because they allow us to reduce the frequency with which a person with dermatitis will need corticosteroid creams.
What is the risk?
These creams were launched in the US in 2000 and in Spain in 2002. Clinical trials conducted prior to approval showed them to be safe. However, in the years following their launch, isolated cases of lymphoma and skin cancer were reported in children and adults treated with these medicines. It was therefore decided in 2006 to add this information to the package insert.
The causal relationship between topical calcineurin inhibitors and cancer has not yet been demonstrated.
The causal relationship between the creams and the appearance of these cancers has not been proven. The fact that people who used these creams developed cancer is not necessarily due to the creams; in fact, the incidence of lymphoma in people who use these creams is the same as in the normal population (article on this subject).
The frequency of lymphomas is the same among people treated with calcineurin inhibitors as in the normal population.
To date, several studies have been performed in search of this causal relationship and to date, after more than 10 years of experience, no study has concluded that these lymphomas and skin tumors occurred because of Tacrolimus or Pimecrolimus (as stated in this article published a few months ago).
In fact, opinion articles have recently been published in medical sources and scientific journals calling for a modification of the paragraph in question in the package inserts to avoid the fear it causes in patients and their parents. It is believed that because of this warning, people who could benefit from the positive effects of these drugs do not use them because of unfounded fear.
¿Se pueden utilizar en bebés?

The package inserts for both creams state that they are approved for use from 2 years of age. However, their usefulness in infants is well known to dermatologists. This is supported by the Petite study published in 2015 in Pediatrics, the premier pediatric journal, which demonstrated the safety of these medications (as well as topical corticosteroids) in more than 2000 children over 5 years.
It is safe to use calcineurin inhibitors in children under 2 years of age.
Can they be used for other skin diseases?
Strictly speaking these creams are approved exclusively for atopic dermatitis, but they can be safely used on other skin conditions, such as seborrheic dermatitis or rosacea on occasion. If you have any doubts about this, it is best to consult a dermatologist who can indicate the best option in your case.
In conclusion…
Tacrolimus (Protopic®) and pimecrolimus (Elidel®) are creams included in all atopic dermatitis treatment guidelines and we have more than 10 years of experience in their use. Although the package insert is alarming, numerous scientific studies confirm that the risk is not such and that they are an excellent choice for dermatitis of delicate areas, as well as for reducing the need for corticosteroid creams, both in children and adults.